
Education:
BS, University of Kansas, 1985
PhD, Northwestern University, 1990
NIH Postdoctoral Fellow, California Institute of Technology (Dervan), 1990-1992
Professional Experience:
Schlundt Distinguished Professor of Chemistry, 2005-present
Professor of Chemistry, 2001-present
Joint Appointment, Department of Biochemistry 1992-present
Associate Professor of Chemistry, 1998-2001
Assistant Professor of Chemistry, 1993-1998
Professional Activities:
Elected as a Fellow of the American Association for the Advancement of Science (AAAS), 2012
Elected as a Fellow of the American Chemical Society (ACS), 2016
Gold Chalk Award, MU Graduate Professional Council, 2015
Fuldner Faculty Fellow, MU College of Arts and Sciences, 2013 and 2014
Excellence in Education Award, MU Division of Student Affairs, 2014
Invited participant, NASA/NSF Workshop on Alternative Chemistries of Life, Washington, DC April 1-4, 2012
Associate Member, Siteman Cancer Center, Washington University, St. Louis, Medical School, 2023-2026
Editorial Board Member Journal of Biological Chemistry (JBC) journal of the ASBMB, 2019-present
Editorial Advisory Board, Chemical Research in Toxicology, 2000-2010
Editorial Advisory Board, Current Medicinal Chemistry-Anti-Cancer Agents, 2005-2010
Editorial Advisory Board, Sulfur Chemistry, 2004-present
Editorial Advisory Board, Analytical Chemistry Insights, 2006-2018
Member Faculty of 1000 in the area of Drug Discovery and Design. 2013-2016
Program Chair for the Division of Chemical Toxicology of the ACS, 2006-2008
Co-founder and co-organizer of Nucleic Acid Topics Summit Conference, Telluride, CO 2008
Member, Cancer Etiology Study Section of the National Institutes of Health, 2001-2005
Ad hoc Member, Drug Discovery and Molecular Pharmacology Study Section of the NIH, 2008
Ad hoc Member, Bioorganic Natural Products Study Section of the NIH, 1998
Member, American Chemical Society (ACS)
Member, American Association for the Advancement of Science (AAAS)
Member, American Association for Cancer Research (AACR)
Courses taught: Medicinal Chemistry and Chemical Biology (The Organic Chemistry of Drug Design and Drug Action); Organic Reaction Mechanisms; Introductory Organic Chemistry, Advanced Nucleic Acid Chemistry
Research Areas:
Medicinal Chemistry, Chemical Biology, Nucleic Acid Chemistry, Organic Chemistry
The group is investigating the chemical mechanisms by which endogenous cellular processes and pharmacological agents modulate the function of biological macromolecules like enzymes and DNA. We employ the tools of synthetic organic chemistry, physical organic chemistry, biochemistry, biophysics, and molecular biology to characterize the molecular events underlying important biological processes.
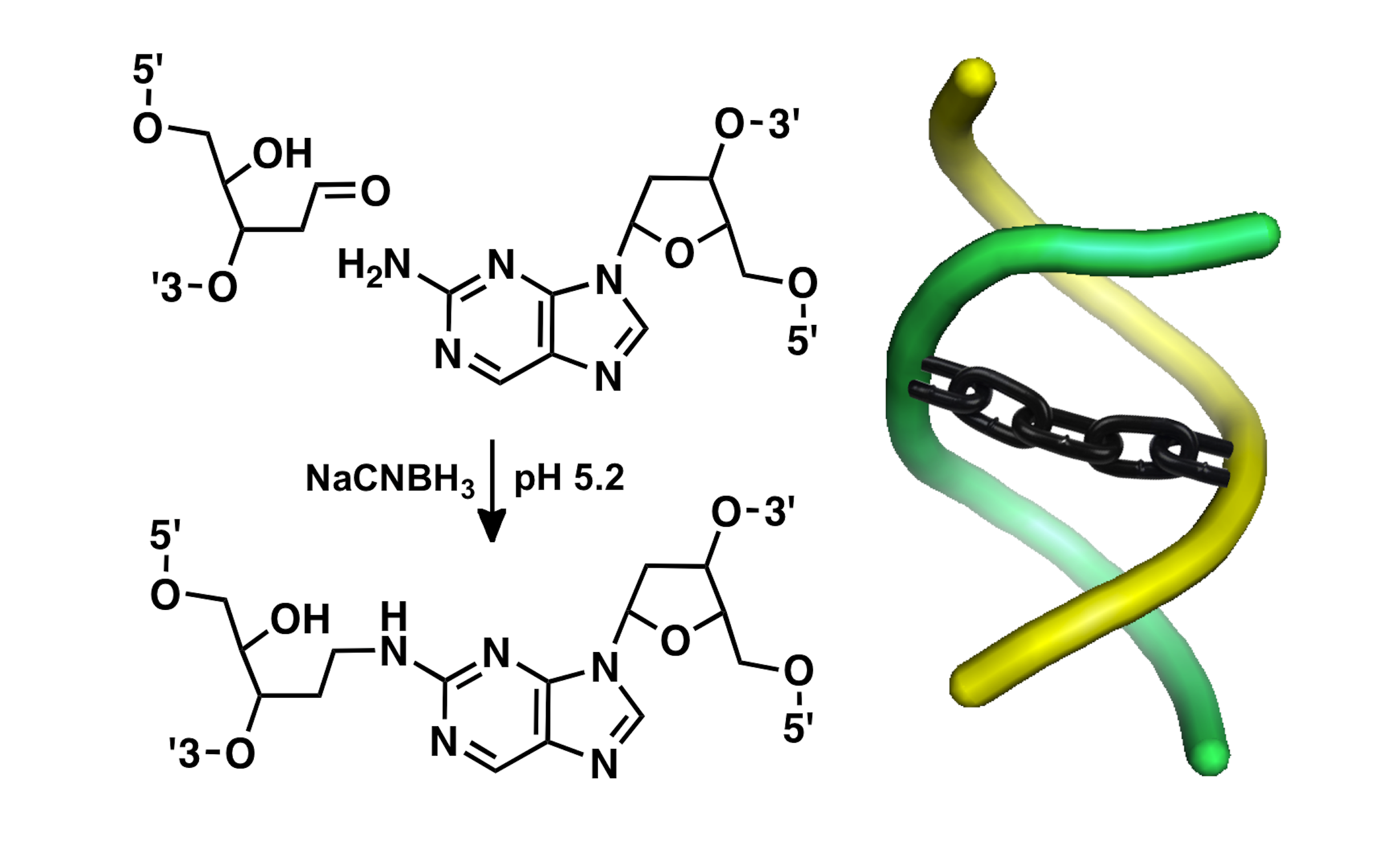
Unavoidable and endogenous cellular DNA damage: a primary driver of human aging, cancer, and neurodegeneration? The information stored in cellular DNA provides the blueprint and operating instructions for all living creatures on earth. Maintaining the integrity of the genetic material is mission-critical for living organisms, yet low levels of DNA damage are an unavoidable consequence of cellular life and may be a primary driver of evolution, human aging, cancer, and neurodegeneration. We are identifying and characterizing important chemical pathways underlying unavoidable, endogenous DNA damage. Specifically, we have identified reactions by which abundant lesions in cellular DNA called abasic sites generate interstrand cross-links that covalently fuse the two strands of the double helix. Endogenous DNA cross-links may be particularly significant in biology because they block the strand separation of double-helical DNA that is required for read-out and replication of the genetic code in cells. Understanding the molecular basis of aging, cancer, and neurodegeneration may lead to advances in personalized, genomic, predictive medicine.
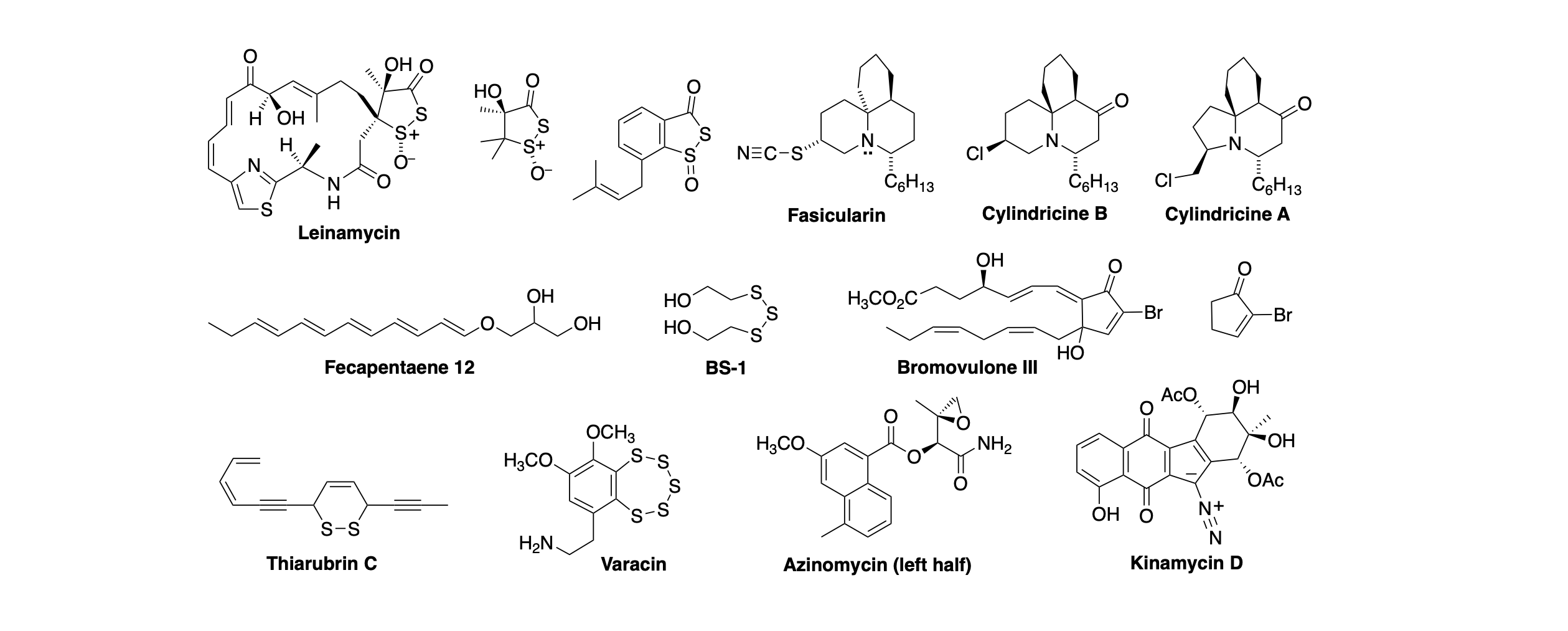
DNA-damaging natural products as a source novel anticancer agents. Cancer is group of diseases characterized by unregulated cell proliferation. DNA-damaging agents can prevent the growth of cancerous tissue by interfering with the DNA replication required for cell division. Interestingly, more than 60% of the anti-infective and anticancer agents in clinical use are natural products (or are derived from natural products). Through the combined power of combinatorial biosynthesis and natural selection, secondary metabolites derived from plants and microorganisms derive potent biological activity from unexpected chemical and biochemical mechanisms. Our work is focused on the chemistry and biology of structurally novel natural products that damage DNA by unusual chemical mechanisms. Some of the natural products that we have studied are shown below.
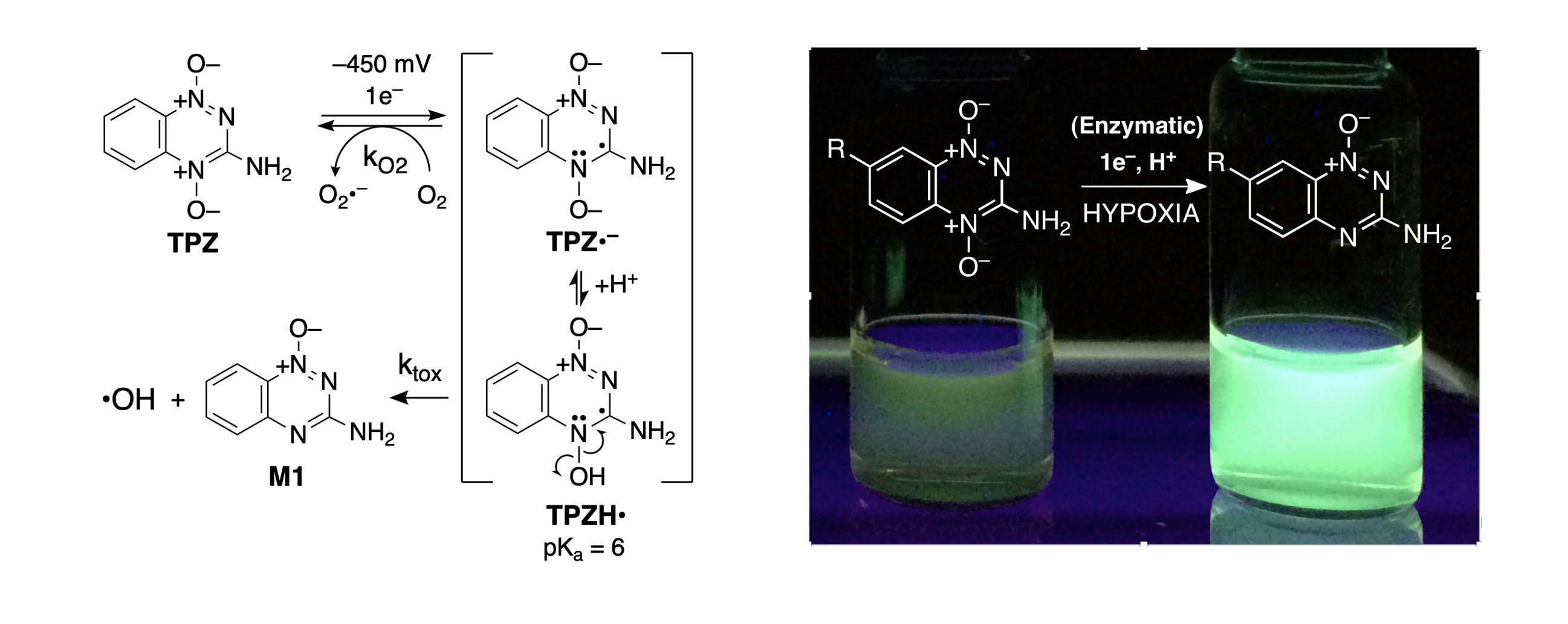
Hypoxia-selective anticancer drugs: exploiting the low-oxygen environment of tumors for the targeted killing of cancer cells. Solid tumors contain significant regions of oxygen-poor (hypoxic) cells. We are studying potential anticancer drugs that selectively generate cell-killing, DNA-damaging reactive intermediates in hypoxic cells. The compound 3-amino-1,2,4-benzotriazine 1,4-dioxide (tirapazamine) is the prototype in this field. Intracellular, one-electron enzymatic reduction of this compound generates an oxygen-sensitive radical intermediate that is simply back-oxidized in normal aerobic tissue but partitions forward to release the DNA-damaging agent hydroxyl radical in hypoxic tissue. Understanding the mechanisms underlying the hypoxia-selective antitumor activity of tirapazamine may enable the development of novel cancer drugs and imaging agents.
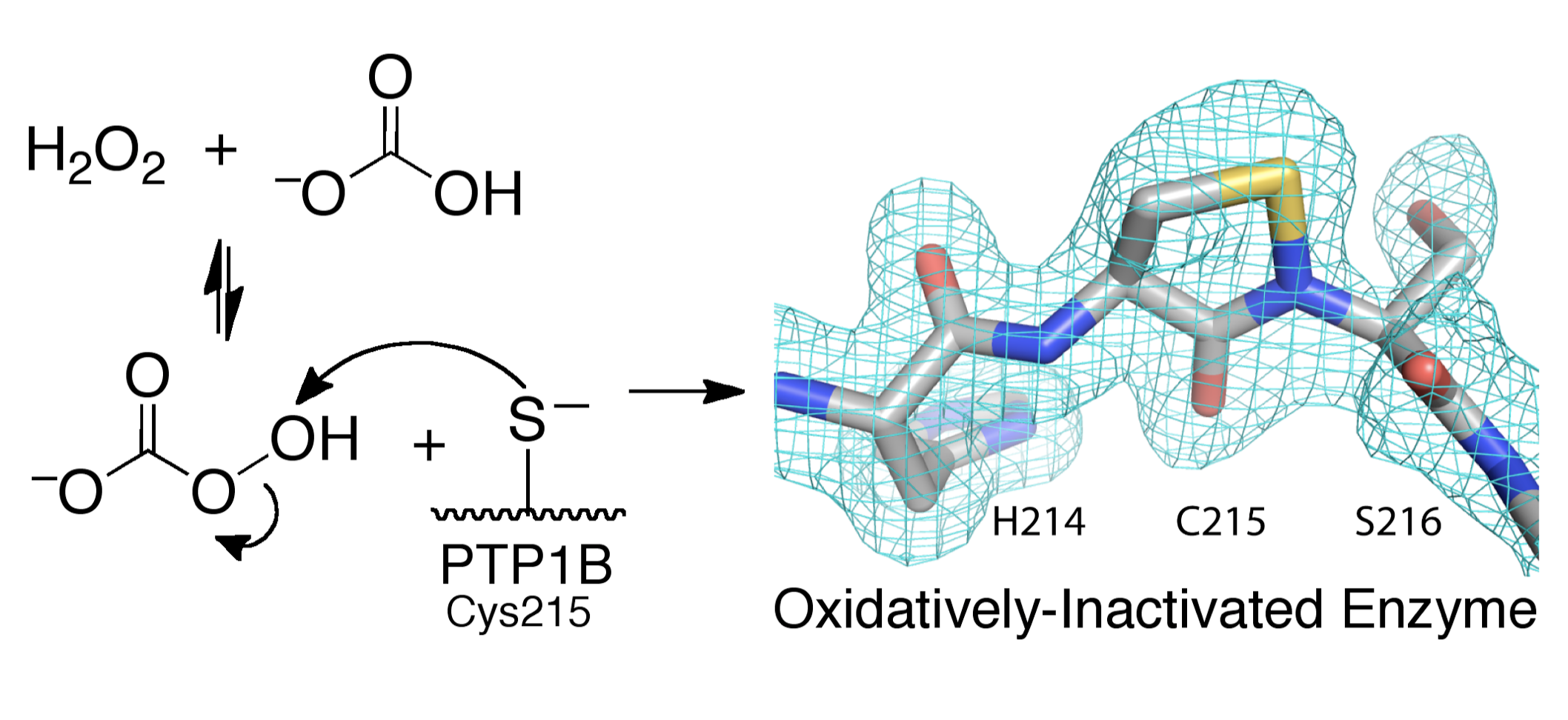
Endogenous, dietary, and pharmacological regulation of protein tyrosine phosphatase activity. The phosphorylation of tyrosine residues on some proteins is an important cellular mechanism for the regulation of protein function in mammalian signal transduction. Protein tyrosine kinases are the enzymes that add phosphoryl groups to target tyrosine residues and protein tyrosine phosphatases (PTPs) are the enzymes that remove these regulatory phosphoryl groups. The regulation of PTP activity plays a critical role in diseases such as cancer and diabetes. Our work characterizes mechanisms by which endogenous (naturally-occurring), dietary, and pharmacological (drug) agents modulate cellular activity of protein tyrosine phosphatases. For example, we showed that peroxycarbonate (derived from the reaction of hydrogen peroxide with intracellular bicarbonate) is a potent inactivator of PTPs. Subsequent work has provided evidence that this mechanism is important in mammalian cells.
The Research Group (from left): Tuhin Haldar, Nathan Price, Amanda Wallace, Garam Shim, Kent Gates, Tanhaul Islam, Anu Gomina, Mia Connor, Jay Jha, Marjan Heidari, Xu Guo, Saosan Binth Md Amin

Repurposing the antihypertensive agent hydralazine as an inhibitor of the base excision repair enzyme APE1. Tanhaul Islam, Venkatrao Nunna, Don Pivithuru Liyanarachchi, Doug Melton, Calvin M. Lewis, and Kent S. Gates. Chem. Res. Toxicol. 2025, 38, 42-45
Ultrafast reaction of the drug hydralazine with apurinic/apyrimidinic sites in DNA gives rise to a stable triazolo[3,4-a]phthalazine adduct. Tanhaul Islam, Garam Shim, Douglas Melton, Calvin D. Lewis, Zhentian Lei, and Kent S. Gates Chem. Res. Toxicol. 2024, 37, 1023-1034
Noncovalent Inhibition and Covalent Inactivation of Proline Dehydrogenase by Analogs of N-propargylglycine. John J. Tanner, Juan Ji, Alexandra N. Bogner, Gary K. Scott, Sagar M. Patel, J. Seravalli, Kent S. Gates, Christopher C. Benz, Donald F. Becker Biochemistry 2024, 63(21), 2855-2867
The intercalator ethidum bromide generates covalent adducts at apurinic/apyrimidinic sites in DNA. Tanhaul Islam, Saosan Binth Md Amin, Kent S. Gates Chem. Res. Toxicol. 2024, 37(11), 1911-1917
Formation and properties of DNA adducts generated by reactions of abasic sites with 1,2-aminothiols including cysteamine, cysteine methyl ester, and peptides containing N-terminal cysteine residues. Anuoluwapo Gomina, Tanhaul Islam, Garam Shim, Zhentian Lei, and Kent S. Gates Chem. Res. Toxicol. 2024, 37, 395-406. doi.org/10.1021/acs.chemrestox.3c00345
Novel processes associated with the repair of interstrand cross-links derived from abasic sites in duplex DNA: roles for the base excision repair glycosylase NEIL3 and the SRAP protein HMCES. Nathan E. Price and Kent S. Gates Chem. Res. Toxicol. 2024, 37, 199-207. doi.org/10.1021/acs.chemrestox.3c00345
N-Methyl-N-Nitrosourea Induced 3¢-Glutathionylated DNA-Cleavage Products in Mammalian Cells. Yin, J.; Gates, K. S.; Wang, Y. Analyt. Chem. 2022, 94, 15595-15603
Effects of local sequence, reaction conditions, and various additives on the formation and stability of interstrand cross-links derived from the reaction of an abasic site with an adenine residue in duplex DNA. Amin, S. B. M.; Islam, T.; Price, N. E.; Wallace, A.; Guo, X.; Gomina, A.; Heidari, M; Johnson, K. M.; Lewis, C. D.; Yang, Z.; Gates, K. S. ACS Omega 2022, 7, 36888-36901
Reconsidering the chemical nature of strand breaks derived from abasic sites in cellular DNA: evidence for 3’-glutathionylation. Jha, J. S.; Yin, J.; Haldar, T.; Yang, Z.; Wang, Y.; Gates, K. S. J. Am. Chem. Soc. 2022, 144, 10471-10482. DOI 10.1021/jacs.2c02703.
Products generated by amine-catalyzed strand cleavage at apurinic/apyrimidinic sites in DNA: New insights from a biomimetic nucleoside model system. Jha, J. S.; Nel; C.; Haldar, T.; Peters, D.; Housh, K.; Gates, K. S.* Chem. Res. Toxicol. 2022, 35, 203-217. DOI: 10.1021/acs.chemrestox.1c00408. This article was selected as an American Chemical Society (ACS) Editors' Choice. The Editor's choice initiative highlights just one article each day from the entire ACS portfolio of journals (60 journals, publishing more than 53,000 articles per year) based on its “potential for broad public interest”.
Unexpected complexity in the products arising from NaOH-, heat-, amine-, and glycosylase-induced strand cleavage at an abasic site in DNA. Haldar, T.; Jha, J. S.; Yang, Z.; Nel, C.; Housh, K.; Cassidy, O. J.; Gates, K. S.* Chem. Res. Toxicol. 2022, 35, 218-232. DOI: 10.11021/acs.chemrestox.1c00409. NOTE: This paper was included in a collection of “highly cited” papers in the journal: https://pubs.acs.org/page/crtoec/vi/high-cited-toxicology?ref=vi_collec.on
Synthesis of DNA duplexes containing site-specific interstrand cross-links via sequential reductive amination reactions involving diamine linkers and abasic sites on complementary oligodeoxynucleotides. Housh, K.; Gates, K. S. Chem. Res. Toxicol. 2021, 34, 2384-2391. doi.org/10.1021/acs.chemrestox.1c00293.
Formation and repair of an interstrand DNA cross-link arising from a common endogenous lesion. Housh, K.; Jha, J. S.; Yang, Z.; Haldar, T.; Johnson, K. M.; Yin, J.; Wang, Y.; Gates, K. S. J. Am. Chem. Soc. 2021, 143, 15344-15357.
Interstrand cross-link formation involving reaction of a mispaired cytosine residue with an abasic site in duplex DNA. Varela, J. G.; Pierce, L. E.; Guo, X.; Price, N. E.; Johnson, K. M.; Yang, Z.; Wang, Y.; Gates, K. S. Chem. Res. Toxicol. 2021, 34, 1124-1132.
Campbell, A. C.; Prater, A. R.; Bogner, A. N.; Quinn, T. P.; Gates, K. S.; Becker, D. F.; Tanner, J. J. Photoinduced covalent irreversible inactivation of proline dehydrogenase by S-heterocycles. ACS Chem. Biol. 2021, 16, 2268-2279.
Structure of a stable interstrand DNA crosslink involving a β-N-glycosyl linkage between an N6-dA amino group and an abasic site. Kellum, Jr., A.; Qiu, D.; Voehler, M.; Martin, W.; Gates, K. S.; Stone, M. P. Biochemistry 2021, 60, 41-52. doi.org/10.1021/acs.biochem.0c00596. For structure, see pdb code: 6xah
Formation and repair of unavoidable, endogenous interstrand cross-links in cellular DNA. Housh, K; Jha, J. S.; Haldar, T.; Binth Md. Amin, S.; Islam, T.; Wallace, A. Gomina, A.; Guo, X.; Nel, C.; Wyatt, J. W.; Gates, K. S. DNA Repair 2021, 98, 103029.
An autoinhibitory role for the GRF zinc finger domain of DNA glycosylase NEIL3. Rodriguez, A. A.; Wojtaszek, J. L.; Greer, B; Haldar, T.; Gates, K. S.; Williams, R. S.; Eichman, B. F. J. Biol. Chem. 2020, 295(46), 15566-15575. doi/10.1074/jbc.RA120.015541.
Unhooking of an interstrand cross-link at DNA fork structures by the DNA glycosylase NEIL3. Imani-Nejad, M.; Housh, K.; Rodriguez, A. A.; Haldar, T.; Kathe, S.; Wallace, S. S.; Eichman, B. F.; Gates, K. S. DNA Repair 2020, 86, 102752.
Structural analysis of pathogenic mutations targeting Glu427 and ALDH7A1, the hotspot residue of pyridoxine-dependent epilepsy. Laciak, A. R.; Korasick, D. A.; Gates, K. S.; Tanner, J. J. J. Inher. Metabol. Dis. 2020, 43(3),635-644.
Covalent modification of the flavin in proline dehydrogenase by thiazolidine-2-carboxylate. Campbell, A. C.; Becker, D. F.; Gates, K. S.; Tanner, J J. ACS Chem. Biol. 2020, 15(4), 936-944. doi.org/10.1021/acschembio.9b00935
Inhibition, crystal structures, and in-solution oligomeric structure of aldehyde dehydrogenase 9A1. Wyatt, J. W.; Korasick, D. A.; Qureshi, I. A.; Campbell, A. C.; Gates, K. S.; Tanner, J. J. Arch. Biochem. Biophys. 2020, 691, 108477.
Structural and Biochemical Consequences of Pyridoxine-Dependent Epilepsy Mutations That Target the Aldehyde Binding Site of Aldehyde Dehydrogenase ALDH7A1. Laciak, A. R.; Korasick, D. A.; Wyatt, J. W.; Gates, K. S.; Tanner, J. J. FEBS J. 2020, 287, 173-189.
Selective covalent capture of a DNA sequence corresponding to a cancer-driving C>G mutation in the KRAS gene by a chemically reactive probe: optimizing a cross-linking reaction with non-canonical duplex structures. Guo, X.; Imani-Nejad, M.; Gu, L.-Q.; Gates, K. S. RSC Adv. 2019, 9(56), 32804-32810.
Facile generation of duplexes containing a chemically-defined, site-specific, interstrand DNA-DNA cross-link derived from reaction of the non-canonical nucleobase 2-aminopurine with an abasic site. Imani Nejad, M.; Price, N. E.; Haldar, T.; Lewis, C.; Wang, Y.; Gates, K. S. ACS Chem. Biol. 2019, 14(7), 1481-1489.
Preparation and purification of oligodeoxynucleotide duplexes containing a site-specific, reduced, chemically-stable covalent interstrand cross-link between a guanine residue and an abasic site. Imani Nejad, M.; Guo, X.; Housh, K.; Nel, C.; Yang, Z.; Price, N. E.; Wang, Y.; Gates, K. S. Methods Mol. Biol. 2019, 1973, 163-175 (doi.org/10.1007/978-1-4939-9216-4_10).
Enzyme-activated generation of reactive oxygen species from heterocyclic N-oxides under aerobic and anaerobic conditions and its relevance to hypoxia-selective prodrugs. Shen, X.; Gates, K. S. Chem. Res. Toxicol. 2019, 32, 348-361.
PATENT: Nucleic acid sequence and capture by formation of an abasic site-derived cross-link. Gates, Kent; Gu, Li-Qun; Nejad, Maryam Imani; Shi, Ruicheng; Zhang, Xinyue; U.S. Pat. Appl. Publ. (2019), US20190271029 A1 20190905
Generation and single-molecule characterization of a sequence-selective covalent cross-link mediated by mechlorethamine at a C-C mismatch in duplex DNA for discrimination of a disease-relevant single nucleotide polymorphism. Shi, Ruicheng; Nejad, Maryam Imani; Zhang, Xinyue; Gu, Li-Qun; Gates, Kent S. Bioconjugate Chem. 2018, 29(11), 3810-3816.
Exploiting the inherent photophysical properties of the major tirapazamine metabolite in the development of profluorescent substrates for enzymes that catalyze bioreductive activation of hypoxia-selective anticancer prodrugs. Shen, X.; Laber, C. H.; Sarkar, U.; Galazzi, F.; Johnson, K. M.; Mahieu, N.; Barnes, C. L.; Baker, G. A.; Gates, K. S. J. Org. Chem. 2018, 83, 3126-3131. DOI: 10.1021/acs.joc.7b03035
Oxidative activation of leinamycin E1 triggers alkylation of guanine residues in double-stranded DNA. Imani-Nejad, M.; Yang, D.; Shen, B.; Gates, K. S. Chem. Commun. 2018, 54, 256-259. DOI: 10.1039/c7cc08482j
Single Locked Nucleic Acid-Enhanced Nanopore Genetic Discrimination of Pathogenic Serotypes and Cancer Driver Mutations. Tian, K.; Chen, X.; Luan, B.; Singh, P.; Yang, Z.; Gates, K. S.; Lin, M. L.; Mustapha, A.; Gu, L.-Q. ACS Nano 2018, 12(5), 4194-4205. DOI: 10.1021/acsnano.8b01198
What is the potential of nanolock- and nanocross-nanopore technology in cancer diagnosis? Gu, L.-Q.; Gates, K. S.; Wang, M. X.; Li, G. Exp. Rev. Mol. Diagnos. 2018, 18, 113-117.
Interstrand cross-links arising from strand breaks at true abasic sites in duplex DNA. Yang, Z.; Price, N. E.; Johnson, K. M.; Wang, Y.; Gates, K. S. Nucleic Acids Res. 2017, 45, 6486-6493.
Importance of the C-terminus of aldehyde dehydrogenase 7A1 for oligomerization and catalytic activity. Korasick, D. A.; Wyatt, J. W.; Luo, M. Lackiak, A. R.; Ruddraraju, K.; Gates, K. S.; Henzl, M. T.; Tanner, J. J. Biochemistry 2017, 56, 5910–5919.
Nanolock-nanopore facilitated digital diagnostics of cancer driver mutation in tumor tissue. Wang, Y.; Tian, K.; Shi, R.; Gu, A.; Pennella, M.; Alberts, L.; Gates, K. S.; Li, G.; Fan, H.; Wang, M. X.; Gu, L.-Q. ACS Sensors 2017, 2, 975-981.
Replication and repair of a reduced 2’-deoxyguanosine-abasic site cross-link in human cells. Price, N. E.; Li, L.; Gates, K. S.; Wang, Y. Nucleic Acids Res. 2017, 45, 6486-6493.
Covalent allosteric inactivation of PTP1B by an inhibitor-electrophile conjugate. Punthasee, P.; Laciak, A. R.; Cummings, A. H.; Ruddraraju, K. V.; Lewis, S. M.; Hillebrand, R.; Singh, H.; Tanner, J. J.; Gates, K. S. Biochemistry 2017, 56, 2015-2060.
Sequence-specific covalent capture coupled with high-contrast nanopore detection of a disease-derived nucleic acid sequence. Imani Nejad, M.; Shi, R.; Zhang, X., Gu, L.-Q.; Gates, K. S. ChemBioChem. 2017, 18, 1383-1386.
A role for the base excision repair enzyme NEIL3 in replication-dependent repair of interstrand cross-links derived from psoralen and abasic sites. Yang, Z.; Imani Nejad, M.; Gamboa Varela, J.; Wang, Y.; Gates, K. S. DNA Repair 2017, 52, 1-11.
Allylation and alkylation of biologically relevant nucleophiles by diallyl sulfides. Ruddraraju, K. V.; Parsons, Z. D.; Lewis, C. D.; Gates, K. S. J. Org. Chem. 2017, 82, 776-780.
Application of Suzuki-Miyaura and Buchwald-Hartwig cross-coupling reactions to the preparation of substituted 1,2,4-benzotriazine 1-oxides related to the antitumor agent tirapazamine. U. Sarkar, R. Hillebrand, K. M. Johnson, A. H. Cummings, N. L. Phung, A. Rajapakse, H. Zhou, J. R, Willis, C. L. Barnes and K. S. Gates, J. Het. Chem. 2017, 54, 155-160.
A new cross-link for an old cross-linking drug: the nitrogen mustard anticancer agent mechlorethamine generates cross-links derived from abasic sites in addition to the expected drug-bridged cross-links. Imani-Nejad, M.; Johnson, K. M.; Price, N. E. Gates, K. S. Biochemistry 2016, 55(50), 7033-7041.
Effective molarity in a nucleic acid-controlled reaction. Catalano, M. J.; Price, N. E.; and Gates, K. S. Bioorg. Med. Chem. Lett. 2016, 26, 2627-2630.
Sulfone-stabilized carbanions for the reversible covalent capture of a posttranslationally-generated cysteine oxoform found in protein tyrosine phosphatase 1B (PTP1B). Parsons, Z. D.; Ruddraraju, K. V. and Gates, K. S. Bioorg. Med. Chem. 2016, 24, 2631-2640.
Simple, high-yield synthesis of DNA duplexes containing interstrand DNA-DNA cross-links between an N4-aminocytidine residue and an abasic site. Varela, J. G. and Gates, K. S. Curr. Protoc. Nucleic Acid Chem. 2016, 65, 5.16.1-5.16.15
Crystal structure of a nucleoside model of the interstrand cross-link formed by the reaction of 2’-deoxyguanosine and an abasic site in duplex DNA. Catalano, M. J.; Ruddraraju, K.V. and Gates, K. S. Acta Cryst. E. 2016, E72, 624-627.
Chemical structure and properties of interstrand cross-links formed by reaction of guanine residues with abasic sites in duplex DNA. Catalano, M. J.; Liu, S.; Anderson, N.; Yang, Z.; Johnson, K. M.; Price, N. E.; Wang. Y. and Gates, K. S. J. Am. Chem. Soc. 2015, 137, 3933-3945.
Characterization of interstrand DNA-DNA cross-links using the alpha-hemolysin protein nanopore. Zhang, X.; Price, N. E.; Fang, X.; Yang, Z.; Gu, L.-Q. and Gates, K. S. ACS Nano 2015, 9, 11812-11819.
Reactions of 1,3-diketones with a dipeptide isothiazolidin-3-one: Toward agents that covalently capture oxidized protein tyrosine phosphatase 1B. Ruddraraju, K. V.; Parsons, Z. D.; Llufrio, E. M.; Frost, N. L. and Gates, K. S. J. Org. Chem. 2015, 80, 12015-12026.
Mimicking ribosomal unfolding of an RNA pseudoknot in a protein channel. Zhang, X.; Xu, X.; Yang, Z.; Burcke, A. J.; Chen, S.-J.; Gates, K. S.; Gu, L.-Q. J. Am. Chem. Soc. 2015, 137, 15742-15752.
A simple, high-yield synthesis of DNA duplexes containing a covalent, thermally cleavable interstrand cross-link at a defined location. Varela, J. G.; Gates, K. S. Angew. Chem. Int. Ed. Eng. 2015, 54, 7666-7669. (Designated as a hot paper in journal issue #26
The paper above received a write-up in: Nat. Chem. 2015, 7, 537.
Characterization of interstrand DNA-DNA cross-links derived from abasic sites using bacteriophage phi29 DNA polymerase. Yang, Z.; Price, N. E.; Johnson, K. M.; Gates, K. S. Biochemistry 2015, 54 4259-4266.
Diethylaminobenzaldehyde Is a Covalent, Irreversible Inactivator of ALDH7A1. Luo, M.; Gates, K. S.; Henzl, M. T. and Tanner, J. J. ACS Chem. Biol. 2015, 10, 693-697.
Inactivation of protein tyrosine phosphatases by dietary isothiocyanates. Lewis, S. M.; Li, Y.; Catalano, M.; Laciak, A. R.; Singh, H.; Seiner, D. R.; Reilly, T. J.; Tanner, J. J.; Gates, K. S. Bioorg. Med. Chem. Lett. 2015, 25, 4549-4552.
Chemical and structural characterization of cross-links formed between abasic sites and adenine residues in duplex DNA. Price, N. E.; Catalano, M. J.; Liu, S.; Wang, Y. and Gates, K. S. Nucleic Acids Res. 2015, 43, 3434-3441.
Crystal structure of methyl (S )-2-{(R )-4-[(tert-butoxycarbonyl)amino]-3-oxo-1,2-thiazolidin-2-yl}-3-methylbutanoate: a chemical model for oxidized protein tyrosine phosphatase 1B (PTP1B). Ruddraraju, K. V.; Hillebrand, R.; Barnes, C. L. and Gates, K. S. Acta Cryst. E. 2015, E71, 741-743.
Generation of Reactive Oxygen Species Mediated by 1-Hydroxyphenazine, a Virulence Factor of Pseudomonas aeruginosa. Sinha, S.; Shen, X.; Gallazzi, F.; Li, Q.; Zmijewski, J. W.; Lancaster, J. R. Jr.; and Gates, K. S. Chem. Res. Toxicol. 2015, 28, 175-181.
Near-silence of isothiocyanate carbon in 13C-NMR spectra: a case study of allyl isothiocyanate. Glaser, R.; Hillebrand, R.; Wycoff, W.; Camasta, C.; Gates, K. S. J. Org. Chem. 2015, 80, 4360-4369.
Crystal structure of 5-{4-[(2-{2-[2-(2-ammonioethoxy)ethoxy]ethoxy}ethyl)carbamoyl]-4-methoxy-[1,1-biphenyl]-3-yl}-3-oxo-1,2,5-thiadiazolidin-2-ide 1,1-dioxide: a potential inhibitor of the enzyme protein tyrosine phosphatase 1B. Ruddraraju, K. V.; Hillebrand, R.; Barnes, C. L.; Gates, K. S. Acta. Cryst. E 2015, E71, 336-338.
Covalent adduct formation between the antihypertensive drug hydralazine and abasic sites in double- and single-stranded DNA. Melton, D.; Lewis, C.; Price, N. E. and Gates, K. S. Chem. Res. Toxicol. 2014, 27, 2113-2118.
Interstrand DNA-DNA cross-link formation between adenine residues and abasic sites in duplex DNA. Price, N.; Johnson, K. M.; Wang, J.; Fekry, M. I.; Wang, Y.; and Gates, K. S. J. Am. Chem. Soc. 2014, 136, 3483-3490. (doi.org/10.1021/ja410969x)
The article above was one of four spotlighted in the issue of JACS where it appeared (J. Am. Chem. Soc. 2014, 136, 3321) and also was one of seven articles, from all areas of science, highlighted in the Editor`s Choice section of the March 7th issue of Science Magazine (Science 2014, 343, 1058-1059).
Single Molecule Investigation of Ag(I) Interactions with Single Cytosine-, Methylcytosine- and Hydroxymethylcytosine-Cytosine Mismatches in a Nanopore. Yong Wang, Bin-Quan Luan, Zhiyu Yang, Xinyue Zhang, Brandon Ritzo, Kent Gates, and Li-Qun Gu Sci. Reports (www.nature.com/scientificreports) 2014, 4(5883), 1-8 (DOI: 10.1038/srep05883)
DNA double whammy. Gates, K. S. Nat. Chem. 2014, 6, 464-465.
Toward hypoxia-selective DNA-alkylating agents built by grafting nitrogen mustards onto the bioreductively-activated, hypoxia-selective DNA-oxidizing agent 3-amino-1,2,4-benzotriazine 1,4-dioxide (tirapazamine). Kevin M. Johnson, Zachary D. Parsons, Charles L. Barnes, and Kent S. Gates J. Org. Chem. 2014, 79, 7520-7531.
Isotopic labeling experiments that elucidate the mechanism of DNA strand cleavage by the hypoxia-selctive antitumor agent 1,2,4-benzotriazine 1,4-di-N-oxide. Shen, X. Rajapakse, A.; Galazzi, F.; Junnotula, V.; Fuchs-Knotts, T.; Glaser, R.; and Gates, K. S. Chem. Res. Toxicol. 2014, 27, 111-118.
Redox regulation of protein tyrosine phosphatases: methods for kinetic analysis of covalent enzyme inactivation. Parsons, Z. D.; Gates, K. S. Methods Enzymol. 2013, 528, 129-154.
Thiol-dependent recovery of activity from oxidized protein tyrosine phosphatases (PTPs). Parsons, Z. D.; Gates, K. S. Biochemistry 2013, 52, 6412-6423.
Fapy lesions and DNA mutations. Gates, K. S. Nat. Chem. Biol. 2013, 9, 412-413.
Rajapakse, A.; Linder, C.; Morrison, R. D.; Sarkar, U.; Leigh, N. D.; Barnes, C. L.; Daniels, J. S.; Gates, K. S. Enzymatic conversion of 6-nitroquinoline to the fluorophore 6-aminoquinoline selectively under hypoxic conditions. Chem. Res. Toxicol. 2013, 26, 555-563.
On the Formation and Properties of Interstrand DNA-DNA Cross-links Forged by Reaction of an Abasic Site With the Opposing Guanine Residue of 5’-CAp Sequences in Duplex DNA. Johnson, K. M.; Price, N. E.; Wang, J.; Fekry, M. I.; Dutta, S.; Seiner, D. R.; Wang, Y.; Gates, K. S. J. Am. Chem. Soc. 2013, 135, 1015-1025.
Synthesis and characterization of a small analogue of the anticancer natural product leinamycin. Keerthi, K.; Rajapakse, A.; Gates, K. S. Submitted to Bioorg. Med. Chem. 2013, 21, 235-241.
Transferring oxygen isotopes to 1,2,4-benzotriazine 1-oxides forming the corresponding 1,4,-dioxides using the HOF•CH3CN complex. Gatenyo, J.; Johnson, K.; Rajapakse, A.; Gates, K. S.; Rozen, S. Tetrahedron 2012, 68(43), 8942-8944.
Cellular responses to the DNA-damaging natural compound leinamycin. Sinha, P.; Shin, Y.; Hays, A. M.; Gates, K. S.; Sun, D. J. Cancer Sci. Ther. 2012, S8 (open access journal) S8:003. doi:10.4172/1948-5956.S8-003.
DNA cleavage induced by antitumor antibiotic leinamycin and its biological consequences. Viswesh, V.; Hayes, A. M.; Gates, K. S.; Sun, D. Bioorg. Med. Chem. Lett. 2012, 22, 4413-4421.
Generation of DNA-damaging reactive oxygen species via the autoxidation of hydrogen sulfide under physiologically-relevant conditions: chemistry relevant to both the genotoxic and cell signaling properties of H2S. Hoffman, M.; Rajapakse, A.; Shen, X.; Gates, K. S. Chem. Res. Toxicol. 2012, 25, 1609-1615.
Hypoxia-selective, enzymatic conversion of 6-nitroquinoline into a fluorescent helicene: pyrido[3,2-f]quinolino[6,5-c]cinnoline 3-oxide. Rajapakse, A; Gates, K. S. J. Org. Chem. 2012, 77, 3531-3537.
The macrocycle of leinamycin imparts hydrolytic stability to the thiol-sensing 1,2-dithiolan-3-one 1-oxide unit of the natural product. Sivaramakrishnan, S.; Breydo, L.; Sun, D.; Gates, K. S. Bioorg. Med. Chem. Lett. 2012, 22, 3791-3794.
On the reaction mechanism of tirapazamine reduction chemistry: unimolecular N-OH homolysis, stepwise dehydration, or triazine ring-opening. Yin, J.; Glaser, R.; Gates, K. S. Chem. Res. Toxicol. 2012, 25, 634-645.
Electron and spin-density analysis of tirapazamine reduction chemistry. Yin,, J.; Glaser, R.; Gates, K. S. Chem. Res. Toxicol. 2012, 25, 620-633.
Hypoxia-selective, enzymatic conversion of 6-nitroquinoline into a fluorescent helicene: pyrido[3,2-f]quinolino[6,5-c]cinnoline 3-oxide. Rajapakse, A.; Gates, K. S. Submitted to J. Org. Chem. 2012, 77, 3531-3537.
DNA strand cleavage by the phenazine di-N-oxide natural product myxin under both aerobic and anaerobic conditions. Chowdhury, G.; Sarkar, U.; Pullen, S.; William R. Wilson, W. R.; Rajapakse, A.; Fuchs-Knotts, T. and Gates, K. S. Chem. Res. Toxicol. 2012, 25, 195-206.
Non-covalent DNA binding drives DNA alkylation by leinamycin: evidence that the Z,E-5-(thiazol-4-yl)-penta-2,4-dienone moiety of the natural product serves as an atypical DNA intercalator. Fekry, M. I.; Szekely, J.; Dutta, S.; Breydo, L.; Zang, H.; Gates, K. S. J. Am. Chem. Soc. 2011, 132, 17641-17651.
The biological buffer, bicarbonate/CO2, potentiates H2O2-mediated inactivation of protein tyrosine phosphatases. Zhou, H.; Singh, H.; Parsons, Z. D.; Lewis, S. M.; Bhattacharya, S.; Seiner, D. R.; LaButti, J. N.; Reilly, J. N.; Tanner, J. J.; Gates, K. S. J. Am. Chem. Soc. 2011, 132, 15803-15805.
Redox regulation of protein tyrosine phosphatases: Structural and chemical aspects. Tanner, J. J.; Parsons, Z. D.; Cummings, A. H.; Zhou, H.; Gates, K. S. Antioxidants Redox Signaling 2011, 15(1), 77-97.
Synthesis and Crystal Structure of the Azoxydichinyl Helicene, Pyrido[3,2-f]quinolino[6,5-c]cinnoline 5-Oxide Monohydrate. Rajapakse, A.; Barnes, C. L.; Gates, K. S. J. Chem. Cryst. 2011, 41, 1712-1716.
Kinetic Consequences of Replacing the Internucleotide Phosphorus Atoms in DNA with Arsenic. Fekry, Mostafa I.; Tipton, Peter A.; Gates, Kent S. ACS Chem. Biol. 2011, 6, 127-130.
Thiol-Activated DNA Damage By α-Bromo-2-cyclopentenone. Fekry, M. I.; Price, N.; Zang, H.; Huang, C.; Harmata, M.; Brown, P.; Daniels, J. S.; Gates, K. S. Chem. Res. Toxicol. 2011, 24, 217-228.
Synthesis, Crystal Structure, and Rotational Energy Profile of 3-Cyclopropyl-1,2,4-benzotriazine 1,4-Di-N-oxide. Sarkar, U.; Glaser, R. E.; Parsons, Z. D.; Barnes, C. L.; Gates, K. S. J. Chem. Crystallog. 2010, 40, 624-629.
DNA strand cleaving properties and hypoxia-selective cytotoxicity of 7- chloro-2-thienylcarbonyl-3-trifluoromethylquinoxaline 1,4-dioxide. Junnotula, R.; Rajapakse, A.; Arbillaga, L; Lopez de Cerain, A.; Solano, B.; Villar, R.; Monge, A.; Gates, K. S. Bioorganic Med. Chem. 2010, 18, 3125-3132.
Inactivation of protein tyrosine phosphatases by oltipraz and other cancer chemopreventive 1,2-dithiole-3-thiones. Bhattacharya, S.; Zhou, H.; Seiner, D. R.; Gates, K. S. Bioorganic Med. Chem. 2010, 18, 5945-5949.
Characterization of DNA damage induced by a natural product antitumor antibiotic leinamycin in human cancer cells. Viswesh, V.; Gates, K. S.; Sun, D. Chem. Res. Toxicol. 2010, 23, 99-107.
Protection of a single-cysteine redox switch from oxidative destruction: on the functional role of sulfenyl amide formation in the redox-regulated enzyme PTP1B. Sivaramakrishnan, S.; Cummings, A. H.; Gates, K. S. Bioorganic Med. Chem. Lett. 2010, 20, 444-447.
An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals. Gates, K. S. Chem. Res. Toxicol. 2009, 22, 1747-1760. This review was featured on the cover of the November 2009 issue of Chemical Research in Toxicology.
Kent S. Gates, Guest Editor, Thematic Collection on Chemistry and Biology of DNA Damage. Chem. Res. Toxicol. Virtual Issue. 2009, http://pubs.acs.org/page/crtoec/thematic/dna-damage.html
DNA-catalyzed hydrolysis of DNA phosphodiesters. Fekry, M. I.; Gates, K. S. Nature Chem. Biol. 2009, 5, 710-711.
Initiation of DNA strand cleavage by 1,2,4-benzotriazine 1,4-dioxides antitumor agents: Mechanistic insight from studies of 3-methyl-1,2,4- benzotriazine 1,4-dioxide. Junnotula, V.; Sarkar, U.; Sinha, S.; Gates, K. S. J. Am. Chem. Soc. 2009, 130, 1015-1024.
Biologically relevant chemical properties of peroxymonophosphate (=O3POOH). LaButti, J. N.; Gates, K. S. Bioorganic Med. Chem. Lett. 2009, 19, 218-221.
Oxidative inactivation of PTP1B by organic peroxides. Bhattacharya, S.; LaButti, J. N.; Seiner, D. R.; Gates, K. S. Bioorganic Med. Chem. Lett. 2008, 18, 5856-5859.
Evidence for a Morin type intramolecular cyclization of an alkene with a phenylsulfenic acid group in neutral aqueous solution. Keerthi, K.; Sivaramakrishnan, S.; Gates, K. S. Chem. Res. Toxicol. 2008, 21(7), 1368-1374.
Electronic structures and spin topologies of gamma-picolinium radicals. A study of the homolysis of N-methyl-gamma-picolinium and of benzo-, dibenzo-, and naphthoannulated analogs. Glaser, R.; Sui, Y.; Sarkar, U.; Gates, K. S.J. Phys. Chem. A 2008, 112(21), 4800-4814. This paper was featured on the cover of J. Phys Chem A.
Possible mechanisms underlying the antitumor activity of S-deoxyleinamycin. Sivaramakrishnan, S.; Gates, K. S. Bioorganic Med. Chem. Lett. 2008, 18,3076-3080.
Synthesis and Biological Evaluation of New 2-Arylcarbonyl-3-trifluoromethylquinoxaline 1,4-Di-N-oxide Derivatives and Their Reduced Analogues. Solano, B.; Junnotula, V.; Marin, A.; Villar, R.; Burguete, A.; Vicente, E.; Perez-Silanes, S.; Aldana, I.; Monge, A.; Dutta, S.; Sarkar, U.; Gates, K. S. J. Med. Chem. 2007, 50(22), 5485-5492.
DNA Strand Damage Analysis Provides Evidence That the Tumor Cell-Specific Cytotoxin Tirapazamine Produces Hydroxyl Radical and Acts as a Surrogate for O2. Chowdhury, G.; Junnotula, V.; Daniels, J. S.; Greenberg, M. M.; Gates, K. S. J. Am. Chem. Soc. 2007, 129(42), 12870-12877.
Kinetics and Mechanism of Protein Tyrosine Phosphatase 1B Inactivation by Acrolein. Seiner, D. R.; LaButti, J. N.; Gates, K. S. Chem. Res. Toxicol. 2007, 20(9), 1315-1320.
Redox Regulation of Protein Tyrosine Phosphatase 1B (PTP1B) by Peroxymonophosphate (=O3POOH). LaButti, J. N.; Chowdhury, G.; Reilly, T. J.; Gates, K. S. J. Am. Chem. Soc. 2007, 129(17), 5320-5321.
The Chemical Reactions of DNA Damage and Degradation. Kent S. Gates In: Reviews of Reactive Intermediate Chemistry, Platz, M.; Moss, R. A.; Jones, M. Jr., Eds. John Wiley & Sons, New York. 2007, pp 333-378.
Entering the Leinamycin Rearrangement Reaction Via 2-(Trimethylsilyl)ethyl Sulfoxides. Keerthi, K.; Gates, K. S. Org. Biomol. Chem. 2007, 5(10), 1595-1600.
Interstrand Cross-Links Generated by Abasic Sites in Duplex DNA. Dutta, S.; Chowdhury, G.; Gates, K. S. J. Am. Chem. Soc. 2007, 129(7), 1852-1853.
Crystal structures of 3-methyl-1,2,4-benzotriazine 1-oxide and 2-oxide. Junnotula, V.; Sarkar, U.; Barnes, C. L.; Thallapally, P. K.; Gates, K. S. J. Chem. Cryst. 2006, 36(9), 557-561.
Getting Under Wraps: Alkylating DNA in the Nucleosome. Gates, K. S. Nature Chem. Biol. 2006, 2(2), 64-66.
Noncovalent DNA Binding and Oxidative DNA Damage by Fecapentaene-12. Szekley, J.; and Gates, K. S. Chem. Res. Toxicology 2006, 19(1), 117-121.
DNA Damage by Fasicularin. Dutta, S; Abe, H.; Aoyagi, S.; Kibayashi, C.; Gates, K. S. J. Am. Chem. Soc. 2005, 127, 15004-15005.
The article above was discussed in the “Hot Off the Press” section of: Nat. Prod. Rep. 2006, 23(1), 11-14.
A Chemical Model for Redox Regulation of Protein Tyrosine Phosphatase 1B (PTP1B) Activity. Sivaramakrishnan, S.; Keerthi, K.; Gates, K. S. J. Am. Chem. Soc. 2005, 127, 10830-10831.
The article above was highlighted in the “Editor’s Choice” section of Science 2005, 309(July 29), 671-672… and in Chemical and Engineering News 2005, August 1, page 31.
Generation of Reactive Oxygen Species by a Persulfide (BnSSH). Chatterji, T.; Keerthi, K.; Gates, K. S. Bioorganic Med. Chem. Lett. 2005, 15, 3921-3924.
A Fluorimetric Assay for the Spontaneous Release of an N7-Alkylguanine Residue from Duplex DNA. Shipova, K.; Gates, K. S. Bioorganic Med. Chem. Lett. 2005, 15, 2111-2113.
Enzyme-Activated, Hypoxia-Selective DNA Damage by 3-Amino-2-quinoxalinecarbonitrile 1,4-Dioxide. Chowdhury, G.; Kotandenaya, D.; Barnes, C. L.; Gates, K. S. Chem. Res. Toxicol. 2004, 17(11), 1400-1405.
Biologically Relevant Chemical Properties of N7-Alkylguanine Residues in DNA (Review Article). Gates, K. S.; Nooner, T.; Dutta, S. Chem. Res. Toxicol. 2004, 17(7), 839-856.
Chemical Properties of the Leinamycin-Guanine Adduct in DNA. Nooner, T.; Dutta, S.; Gates, K. S. Chem. Res. Toxicol. 2004, 17(7), 942-949.
Synthesis and DNA-Binding Properties of Thiazole Derivatives Related to Leinamycin. Breydo, L; Zang, H.; Gates, K. S. Tetrahedron Lett. 2004, 45, 5711-5716.
Sequence Specificity of DNA Alkylation by the Antitumor Natural Product Leinamycin. Zang, H.; Gates, K. S. Chem. Res. Toxicol. 2003, 16, 1539-1546.
DNA Base Damage by the Antitumor Agent 3-Amino-1,2,4-benzotriazine 1,4-Dioxide (Tirapazamine). Birincioglu, M.; Jaruga, P.; Chowdhury, G.; Rodriguez, H.; Dizdaroglu, M.; Gates, K. S. J. Am. Chem. Soc. 2003, 125(38), 11607-11615.
A Mass Spectrometry Study of Tirapazamine and Its Metabolites: Insights Into the Mechanism of Metabolic Transformations and Characterization of Reaction Intermediates. Zagorevskii, D.; Song, M.; Breneman, C.; Yuan, Y.; Fuchs, T.; Gates, K. S.; Greenlief, C. M. J. Am. Soc. for Mass Spec. 2003, 14(8), 881-892.
Small Molecules That Mimic the Thiol-Triggered Alkylating Properties Seen in the Natural Product Leinamycin. Chatterji, T.; Kizil, M.; Keerthi, K.; Chowdhury, G.; Pospisil, T.; Gates, K. S. J. Am. Chem. Soc. 2003, 125(17), 4996-4997.
The article above was highlighted in the “Editor’s Choice” section of Science 2003, 300(May 2), 703-705.
Reaction of Thiols with 7-Methylbenzopentathiepin. Chatterji, T.; Gates, K. S. Bioorg. Med. Chem. Lett., 2003, 13, 1349-1352.
Activation of Leinamycin by Thiols: A Theoretical Study. Breydo, L.; Gates, K. S. J. Org. Chem. 2002, 67, 9054-9060.
Oxidative DNA Base Damage by the Antitumor Agent 3-Amino-1,2,4-benzotriazine 1,4-Dioxide (Tirapazamine). Kotandeniya, D.; Ganley, B.; Gates, K. S. Bioorg. Med. Chem. Lett. 2002, 12, 2325-2329.
E,E- and Z,E-Thiazol-5-yl-penta-2,4-dienones. Breydo, L.; Barnes, C. L.; Gates, K. S. Acta Cryst. C, 2002, C58, o447-o449.
Photochemical Electron Transfer Reactions of Tirapazamine. Poole, J. S.; Hadad, C. M.; Platz, M. S.; Fredin, Z. P.; Pickard, L.; Guerrero, E. L.; Kessler, M.; Chowdhury, G.; Kotandeniya, D.; Gates, K. S. Photochem. Photobiol. 2002, 75(4), 339-345.
Crystal Structure of 3-Amino-5-methyl-1,2,4-benzotriazine 1-Oxide: Evidence for Formation of a Covalent Attachment Between a Carbon-Centered Radical and the Antitumor Agent Tirapazamine. Fuchs, T.; Barnes, C. L.; Gates, K. S. J. Chem. Crystallog. 2001, 31(7/8), 387-391.
Redox-Activated, Hypoxia-Selective DNA Cleavage by Quinoxaline 1,4-Dioxide. Ganley, B.; Chowdhury, G.; Bhansali, J.; Daniels, J. S.; Gates, K. S. Bioorganic Med. Chem. 2001, 9, 2395-2401.
DNA Alkylation by Leinamycin Can Be Triggered by Cyanide and Phosphines. Zang, H.; Breydo, L.; Mitra, K. Dannaldson, J.; Gates, K. S. Bioorganic Med. Chem. Lett. 2001, 11, 1511-1515.
Thiol-Independent DNA Alkylation by Leinamycin. Breydo, L.; Mitra, K.; Zang, H.; Gates, K. S. J. Am. Chem. Soc. 2001, 123, 2060-2061.
The article above was highlighted in the Science Concentrates section of Chemical and Engineering News 2001, March 5, page 35.
3-Amino-1,2,4-benzotriazine 4-Oxide: A New Product Arising from Bioreductive Metabolism of the Antitumor Agent 3-Amino-1,2,4-benzotriazine 1,4-Dioxide (Tirapazamine). Fuchs, T.; Chowdhary, G.; Barnes, C. L.; Gates, K. S. J. Org. Chem. 2001, 66, 107-114.
DNA Binding and Alkylation by the "Left Half" of Azinomycin B. Zang, H.; Gates, K. S. Biochemistry 2000, 39, 14968-14975.
Mechanisms of DNA Damage by Leinamycin. Gates, K. S. Chem. Res. Toxicol. 2000, 13, 953-956.
Thiol-Dependent DNA Cleavage by 3H-1,2-Benzodithiol-3-one 1,1-Dioxide. Breydo, L.; Gates, K. S. Bioorganic Med. Chem. Lett. 2000, 10, 885-889.
Covalent Modification of DNA by Natural Products. Kent S. Gates In: Comprehensive Natural Products Chemistry, Volume 7, Chapter 14. Barton, D., Nakanishi, K., Meth-Cohn, O. Eds.; Kool, E. T., Volume Ed. Pergamon Press, Oxford. 1999; pp 491-552 (For a review of this book, see: J. Nat. Prod. 2000, 63(2), 291-291)
Crystal Structure of Methyl trans-3-[(2-(methoxycarbonyl)phenyl)sulfinyl] Acrylate: A Product Resulting from Trapping of a Sulfenic Acid by Methyl Propiolate. Mitra, K.; Barnes, C. L.; Gates, K. S. J. Chem. Crystallogr. 1999, 29(10), 1133-1136.
Photosensitization of Guanine-Specific DNA Damage by a Cyano-Substituted Quinoxaline Di-N-Oxide. Fuchs, T.; Gates, K. S.; Hwang, J.-T.; Greenberg, M. M. Chem. Res. Toxicol. 1999, 12(12), 1190-1194.
Reaction of the Hypoxia-Selective Antitumor Agent Tirapazamine with a C1'-Radical in Single-Stranded and Double-Stranded DNA: The Drug and Its Metabolites Can Serve As Surrogates for Molecular Oxygen in Radical-Mediated DNA-Damage Reactions. Hwang, J.-T.; Greenberg, M. M.; Fuchs, T.; Gates, K. S. Biochemistry 1999, 38(43), 14248-14255.
Chemistry of Thiol-Dependent DNA Damage by the Antitumor Antibiotic Leinamycin. Mitra, K.; Gates, K. S. Recent Res. Devel. Organic Chem. 1999, 3, 311-317.
Direct Evidence for Bimodal DNA Damage Induced by Tirapazamine. Daniels, J. S., Gates, K. S., Tronche, C., Greenberg, M. M. Chem. Res. Toxicol. 1998, 11(11), 1254-1257.
Total Synthesis and DNA-Cleaving Properties of Thiarubrine C. Wang, Y.; Koreeda, M.; Chatterji, T.; Gates, K. S. J. Org. Chem. 1998, 63(24), 8644-8645.
Photochemical DNA Cleavage by the Antitumor Agent 3-Amino-1,2,4-benzotriazine 1,4-Dioxide (Tirapazamine, SR4233). Daniels, J. S.; MacGillivray, L. R.; Gates, K. S. J. Org. Chem. 1998, 63, 10027-10030.
Crystal Structure of 3H-1,2-Benzodithiol-3-one 1-Oxide. Behroozi, S.J.; Barnes C.L.; Gates, K.S., J. Chem. Crystallog. 1998, 28(9), 689-691.
DNA Cleavage by 7-Methylbenzopentathiepin: A Simple Analog of the Antitumor Antibiotic Varacin. Chatterji, T.; Gates, K. S. Bioorg. Med. Chem. Lett. 1998, 8, 535-538.
Synthesis and Structure of Functionalized Derivatives of the Cleft-Shaped Molecule Dithiosalicylide. Mitra, K.; Pohl, M.; Barnes, C. L.; Gates, K. S., J. Org. Chem. 1997, 62, 9361-9364.
Oxidative DNA Cleavage by Leinamycin and Simple 1,2-Dithiolan-3-one 1-Oxides: Evidence for Thiol-Dependent Conversion of Molecular Oxygen to DNA-Cleaving Oxygen Radicals Mediated by Polysulfides. Mitra, K.; Kim, W.; Gates, K. S., J. Am. Chem. Soc. 1997, 119, 11691-11692.
The article above was highlighted in the Science Concentrates section of Chemical and Engineering News 1997, Dec. 8, page 23… and in the “Hot of the Press” section of Nat. Prod. Rep. 1998, 15(1), iii-iv.
Evidence for Thiol-Dependent Production of Oxygen Radicals by 4-Methyl-5-pyrazinyl-3H-1,2-Dithiole-3-thione: Possible Relevance to the Anticarcinogenic Properties of 1,2-Dithiole-3-thiones. Kim, W.; Gates, K. S. Chem. Res. Toxicol. 1997, 10, 296-301.
Reactions of 3H-1,2-Benzodithiol-3-one 1-Oxide with Amines and Anilines. Kim, W.; Dannaldson, J.; Gates, K. S., Tetrahedron Lett. 1996, 37, 5337-5340.
DNA Cleavage by the Antitumor Agent 3-Amino-1,2,4-benzotriazine 1,4-Dioxide (SR4233): Evidence for Involvement of Hydroxyl Radical. Daniels, J.S; Gates, K.S., J. Am. Chem. Soc. 1996, 118, 3380-3385.
1,2-Dithiolan-3-one 1-Oxides: Thiol-Activated DNA-Cleaving Agents Structurally Related to Antitumor Antibiotic Leinamycin. Behroozi, S.J.; Kim, W.; Dannaldson, J.; Gates, K.S. Biochemistry 1996, 35, 1768-1774.
The article above was discussed in: Chemical and Engineering News 1996, Feb. 26, 38.)
The Reaction of n-Propanethiol With 3H-1,2-Benzodithiol-3-one 1-Oxide and 5,5-Dimethyl-1,2-dithiolan-3-one 1-Oxide: Studies Related to the Reaction of Antitumor Antibiotic Leinamycin With DNA. Behroozi, S.B.; Kim, W.; Gates, K.S. J. Org. Chem. 1995, 60, 3964-3966.
Novel Syntheses of Dithiosalicylide. Mitra, K.; Gates, K.S. Tetrahedron Lett. 1995, 36, 1391-1394.