
Education:
BA, Franklin and Marshall College, 1989
PhD, Stanford University, 1995
Professional Experience:
Associate Dean for Research, College of Arts and Science, 2021-present
Department Chair, University of Missouri, 2016-2021
Professor, University of Missouri, 2014-present
Associate Chair for Graduate Studies, University of Missouri, 2007-2016
Associate Professor, University of Missouri, 2006-2014
Assistant Professor, University of Missouri, 2003-2006
Assistant Professor, Pennsylvania State University, 1997-2003
NIH Post-doctoral Fellow, Columbia University, 1995-1997
Research Emphasis:
Fluorescent Sensing ; Bioorganic Chemistry; Molecular Recognition
Research:
Our group has been involved in the preparation of fluorescent sensors for detection of biologically important organic compounds in aqueous media. Fluorescent sensors are compounds which produce visible fluorescence in the presence of a target molecule (analyte). Such sensors have been used to visually trace the presence of certain analytes in and around cells. These studies have proven to be invaluable for the elucidation of cellular mechanisms by giving real-time information about the environment of a cell in a non-destructive manner. However, biologically useful sensors for organic compounds have lagged behind those for metal ions and cellular conditions such as pH and pO2. The main challenge in preparing sensors for organic compounds is obtaining specific, high affinity recognition of the analyte of interest in the complex media of the cell.
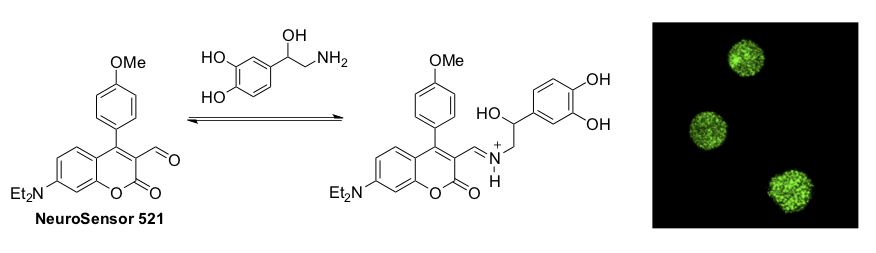
Sensors for Neurotransmitters
Another emphasis of the Glass groups is the preparation of fluorescent sensors for neurotransmitters. With such sensors, neurochemists can trace neurotransmitters in and around neurons and study their functions. We have designed such compounds, termed NeuroSensors, that respond to dopamine and norepinephrine and have shown that they function in live cells.
We have recently expanded on this concept to produce fluorescent chemical logic gates that can be used to detect neurotransmitters as they are being released into the synapse. In the example below, the sensor only responds to the presence of Zinc ion, glutamate, and the pH jump associated with exocytosis. These sensors can be used to study neurochemical release from specialized neursons.
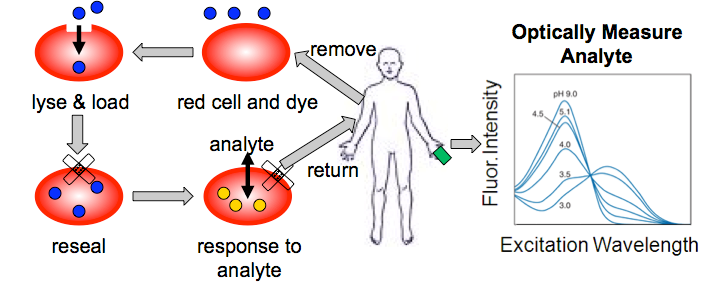
Blood Analyte Detection
One approach to practically useful fluorescent sensing involves the preparation of near infrared (NIR) sensors for blood analytes. These sensors can be read through the skin for completely non-invasive measurement of analytes such as blood pH (among other possible applications). The major problem with this method is the short lifetime of such sensor molecules in blood. We will overcome this challenge by encapsulating the sensors in red blood cells ghosts. Ghosting is a well-known technique that involves removing blood from a patient, opening a lysis pore in the red cell surface and loading the sensor. Resealing the red cells and reinjecting them into the patient will allow the sensor to last much longer than if it was free in serum.
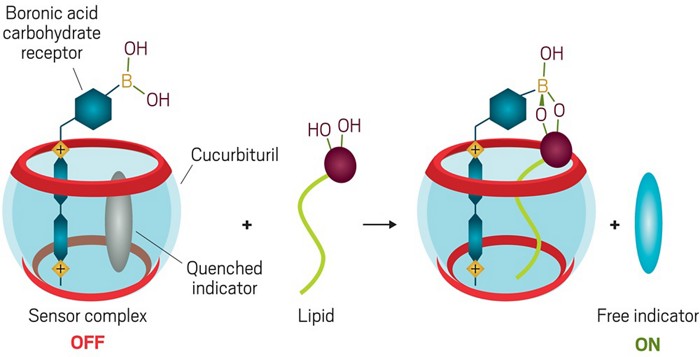
Sensors for Bioactive Lipids
We are also interested the preparation of water-soluble hydrophobic cleft compounds as receptors for bioactive lipids. We have recently used cucurbiturils to assemble sensors for glycolipids. We anchor a carbohydrate sensor in the cucurbituril by attaching it to a viologen with high affinity for the cavity. Then, we insert a fluorescent indicator which is quenched by the viologen. Binding a glycolipid, displaces the indicator, which is then fluorescent.
Smith, M. R.; Zhang, L.; Jin, Y.; Yang, M.; Bade, A.; Gillis, K. D.; Sadden, J.; Naidu Bypaneni, R.; Glass, T. E.*; Lin, H.* "A Turn-On Fluorescent Amino Acid Sensor Reveals Chloroquine’s Effect on Cellular Amino Acids via Inhibiting Cathepsin L" ACS Cent. Sci. 2023, 9, 980-991.
Zhang, L.; Liu, X.A.; Gillis, K.D.; Glass, T. E. “A High Affinity Fluorescent Catecholamine Sensor: Application to Monitoring Norepinephrine Exocytosis” Angew. Chem. Int. Ed. 2019, 58, 7611-7614.
Hettie, K. S.; Klockow, J. L.; Glass, T. E.; Chin, F.T. “Near-Infrared Fluorescent Rosol Dye Tailored toward Lymphatic Mapping Applications” Analytical Chemistry 2019, 91, 3110-3117.
Xu, M.; Kelley, S.; and Glass, T. E. “A Multi-Component Sensor System for Detection of Amphiphilic Compounds” Angew. Chem. Int. Ed. 2018, 57, 12741-12744.
Yin, C.; Huo, F.; Cooley, N. P.; Spencer, D.; Bartholomew, K.; Barnes, C. L.; and Glass, T. E. “A Two-Input Fluorescent Logic Gate for Glutamate and Zinc” ACS Chem. Neurosci. 2017, I, 1159-1162.
Yang, Y.; Huo, F.; Yin, C.; Xu, M.; Hu, Y.; Chao, J.; Zhang, Y.; Glass, T. E.; and Yoon, J. "A Novel Method for the Synthesis of 1,2-Benzisoxazoline-3-one and Its Application to Hypochlorite Recognition" J. Mater. Chem. B, 2016, 4, 5101-5104
Hettie, K. S.; and Glass, T. E. “Turn-On Near-Infrared Fluorescent Sensor for Selectively Imaging Serotonin” ACS Chem. Neurosci. 2016, 7, 21-25.
Klockow, J. L.; Hettie, K. S.; Secor, K. E.; Barman, D. N.; and Glass, T. E. “Tunable Molecular Logic Gates Designed for Imaging Released Neurotransmitters” Chem. Eur. J. 2015, 21, 11446–11451.
Tran, T. M.; Alan, Y. A.; and Glass, T. E. “A highly selective fluorescent sensor for glucosamine ” Chem. Commun. 2015, 51, 7915-7918.
Hettie, K. S.; and Glass, T. E. “Coumarin-3-Aldehyde as a Scaffold for the Design of Tunable PET- 5 Modulated Fluorescent Sensors for Neurotransmitters” Chem. Eur. J. 2014, 20, 17488-17499.
Hettie, K. S. Klockow, J. L.; and Glass, T. E. “Three-input logic gates with potential applications for neuronal imaging” J. Am. Chem. Soc. 2014, 136, 4877-4880.
Ren, C.; Lee, J. S.; Glass, T. E. “A novel fluorescent sensor for hydrophobic amines in aqueous solution” Supramol. Chem., 2014, 26, 607-611.
Cooley, C. M.; Hettie, K. S.; Klockow, J. L.; Garrison, S.; and Glass, T. E. “A Selective Fluorescent Chemosensor for Phosphoserine” Org. Biomol. Chem. 2013, 11, 7387 - 7392.
Klockow, J. L.; Hettie, K. S. and Glass, T. E. “ExoSensor 517: A Dual-Analyte Fluorescent Chemosensor for Visualizing Neurotransmitter Exocytosis” ACS Chem. Neurosci. 2013, 4, 1334-1338.
Hettie, K. S.; Liu, X.; Gillis, K.D.; and Glass, T. E. “Selective Catecholamine Recognition with NeuroSensor 521: A Fluorescent Sensor for the Visualization of Norepinephrine in Fixed and Live Cells” ACS Chem. Neurosci. 2013, 4, 918-923.
Klockow, J. L.; Glass, T. E. “Development of a Fluorescent Chemosensor for the Detection of Kynurenine” Org. Lett. 2013, 15, 235-247.
Zhu, W; Zajicek, J. L; Tillitt, D. E.; Glass, T. E. “TanA: a fluorogenic probe for thiaminase activity” Anal. Methods 2013, 5, 446-448.
Avetta, C. T.; Shorthill, B. J.; Ren, C.; Glass, T. E. “Molecular Tubes for Lipid Sensing: Tube Conformations Control Analyte Selectivity and Fluorescent Response” J. Org. Chem. 2012, 77, 851-857.