
Education:
- 2008 Ph.D. in Chemistry, Department of Chemistry, Stanford University, CA, USA
- 2002 M.Sc. in Chemistry, University of Central Florida, FL, USA
Professional Experience:
- 2020-present Associate Professor, Chemistry Department, University of Missouri, Columbia
- 2011-present Director of the Laboratory for Bioorganic Chemistry and Molecular Imaging, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), Switzerland
- 2008-2011 Postdoctoral Researcher, University of California, Berkeley, CA, USA
Our laboratory develops novel chemical tools to address important questions in biology and medicine with the aim of advancing our understanding of underlying mechanisms of human diseases. Currently, we are focusing on four major projects described below.
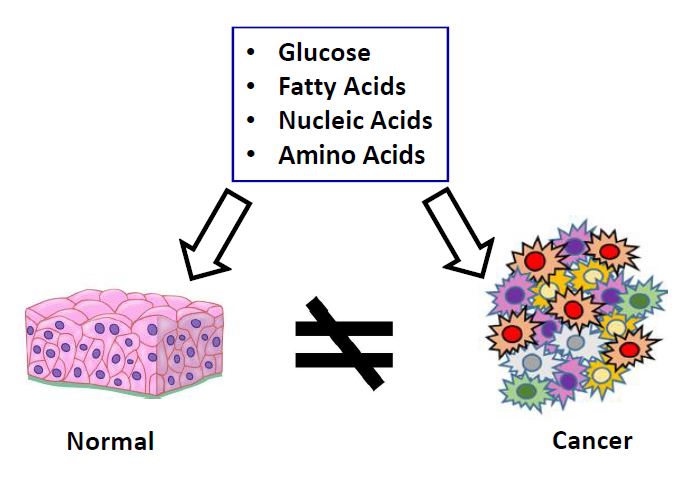
Project 1. Cancer Metabolic Reprogramming
Cancer metabolic reprogramming has been recently recognized as an important hallmark of cancer. It relies of the fact that cancer tissue possesses several important metabolic features, such as differential utilization of many essential metabolites. Cancer metabolic reprogramming is known to be required for malignant transformation, tumor development, invasion and metastasis. Its complex and dynamic nature has been recognized as one of the major challenges for effective cancer treatment. Therefore, a better understanding of metabolic dependencies in specific tumor types can provide a path for improved cancer treatments.
However, no efficient methodologies currently exist that allow noninvasive imaging and quantification of the uptake of essential metabolites in animal models of disease. Current strategies rely on either nuclear imaging techniques such as PET/SPECT/MRI or endpoint ex vivo quantification of metabolites (ex. MS). All of these methods have significant limitations, resulting in a lack of understanding of tumor metabolism and, consequently, poor predictability and efficacy of cancer therapies.
To address the unmet need for nutrient uptake imaging tools, we decided to develop a novel platform based on a combination of versatile “click” chemistry reactions with noninvasive, ultrasensitive bioluminescent imaging techniques. The applications of the novel platform are focused on tumor uptake measurements of glucose, fatty acids, amino acids, and nucleosides previously reported to play important roles in cancer metabolism. The results will lead to the generation of novel, effective treatments; therefore, this novel platform has high clinical applicability. Due to its versatile nature, application of the platform can be expended to studies of many other important human pathologies in which changes in metabolism play a key role, such as diabetes, neurodegenerative diseases, nonalcoholic steatohepatitis, and many others. Please see the first demonstration of the platform recently published in Nature Methods.

Project 2. Development of new tools for probing mitochondrial activity in cells and living animals
In addition to studies of metabolite uptake, we are developing a novel set of tools for studies of mitochondrial functions, which are also known to play a central role in cancer metabolism. We will then use this novel tool to investigate the metabolic reprogramming of different types of cancers.
Mitochondria are essential organelles that provide eukaryotic cells with energy in the form of ATP generated through aerobic respiration. Electrons harvested from the oxidation of carbon food sources are utililized to pump protons across the inner membrane and store the energy in a proton gradient, which is then used to produce ATP through chemiosmosis. Aside from this critical role of ATP production, mitochondria are also important in multiple biological processes including cell differentiation, cell cycle control, cell survival, neuronal protection, and aging.
Mitochondrial dysfunction contributes to a remarkably wide range of human diseases including cancer, Alzheimer’s disease, Parkinson’s disease, diabetes, ischemia perfusion injury, steatohepatitis, sepsis, Huntington’s disease, and many others. As more information associating mitochondrial dysfunction with human diseases emerges, the development of new tools to interrogate this important organelle becomes increasingly important. Since it is not possible to mimic the complexity of these diseases in cell culture, many animal models that closely reflect these important human pathologies have recently been developed. Mitochondrial membrane potential (ΔΨm) is one of the main indicators of mitochondrial function but its direct role in many human pathologies still remain illusive due to the lack of tools for the sensitive, noninvasive, longitudinal, and nonradioactive imaging of ΔΨm levels both in vitro and in vivo. This issue represents a major obstacle to our understanding of the role of this important function in a range of important human diseases, making the drug discovery process complex and less efficient. To address this unmet need, our lab develops novel chemical biology tools that allow noninvasive longitudinal imaging and quantification of ΔΨm levels both in vitro and in vivo. The approach is based on combination of sensitive bioluminescent imaging and a powerful “click” reaction chemical tool and has wide applicability in the field of biomedical research.
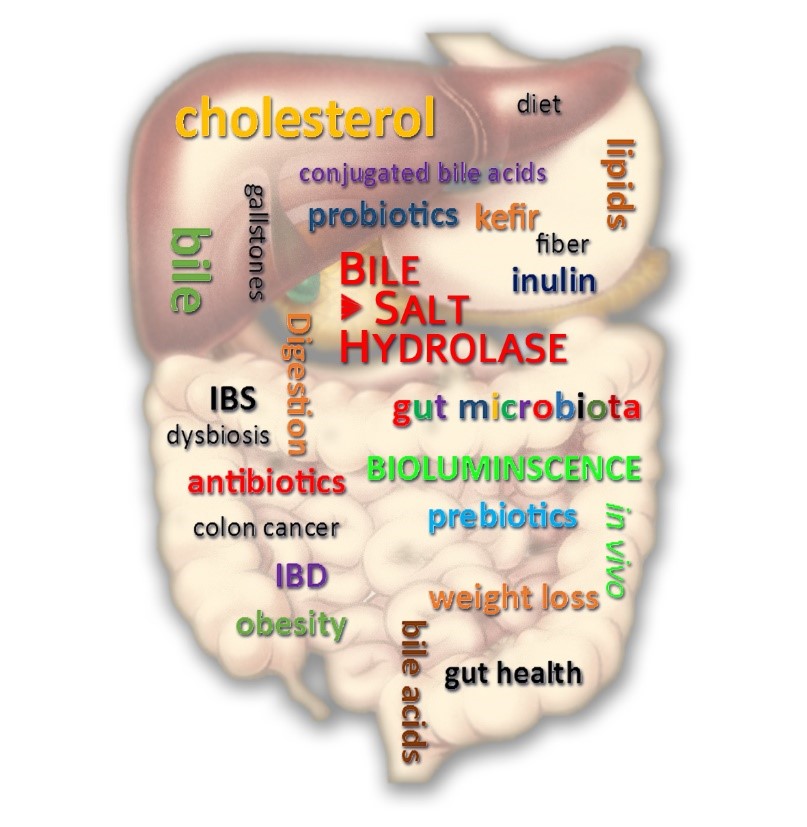
Project 3. Novel tools to unravel enzymatic functions of gut microbiota
The gut microbiota plays a major role in human health and has been characterized as an extremely dynamic and chemically diverse community. Gut microbes significantly impact host physiology by influencing host homeostasis including metabolism, neurobiology, and immune function. The microbiome-produced enzymes play central role in regulation of many essential functions of gut microbiome. For example, bile salt hydrolase (BSH) is thought to play a central role in human health and is responsible for modulation of multiple host signaling pathways, bile detoxification, and gastrointestinal persistence of bacteria. Despite the importance of BAs in host health and disease, the underlying mechanisms by which the gut microbiota enzymes drive their composition and modification are largely unknown. Another important family of gut microbiota enzymes is nitroreductase (NTR) that is known to metabolize nitrosubstituted compounds and quinones using NADH or NADPH as reducing agents. They are important for the development of novel antibiotics being the main target for the treatment of infections caused by bacteria, e.g. Helicobacter pylori, Mycobacterium tuberculosis and by parasites, e.g. Giardia, Trypanosoma and Entamoeba. NTR enzymatic activity in gut microbiota is also linked to carcinogen production and etiology of colorectal cancer.
However, monitoring of enzymatic activity in the gastro-intestinal tract is very challenging given the unique chemical environment, variable distribution of the microbiota, and highly dynamic nature of the microbiota. Currently, no methods exist for noninvasive real-time evaluation of enzymatic activities of the gut microbiota in its intact environment. The existing methods involve ex vivo evaluation of fecal samples, studies of isolated cultured bacteria (in vitro tests), and in silico methods. However, all of these approaches have significant drawbacks for assessment of the composition and function of the microbiota.
To address this unmet need, we developed a novel quantitative optical readout-based method that is bereft of these disadvantages. The design of the method is based on bioluminescence imaging (BLI) and caged-luciferin approach that relies on “caging” luciferin with a small chemical group. First, we adopted this strategy to create a novel assay for non-invasive real-time imaging of NTR activity of gut microbiota (PLOS One, 2015). Next, developed a set of novel “caged” luciferin probes for real-time non-invasive monitoring of BSH activity in bacteria, mice, humans, and clinical samples. Using this assay, we showed for the first time that consumption of particular prebiotics increase BSH activity of the gut microbiota. We also demonstrated successfully application of this novel tool as non-invasive diagnostic test to predict the clinical status of inflammatory bowel disease (IBD) patients. This work represents the first application of functional bioluminescent probes in humans paving the path for clinical translation of these powerful tools for various clinical applications (Science Advances, in press).
Scientific art is created by Marina Resnyanskaya
Yevtodiyenko, A.; Bazhin, A.; Khodakivskyi, P.; Godinat, A.; Budin, G.; Maric, T.; Pietramaggiori, G.; Scherer, S.; Kunchulia, M.; Eppeldauer, G.; Polyakov, S.V.; Francis, K.P.; Bryan, J.N.; Goun, E.* Portable bioluminescent platform for in vivo monitoring of biological processes in non-transgenic animals. Nat. Commun. 2021, accepted
Khodakivskyi, P.V.; Lauber, C.L; Yevtodiyenko, A.; Bazhin, A.A.; Bruce, S.; Ringel-Kulka, T.; Ringel, Y; Betrisey, B.; Torres, J.; Hu, J.; Chou, C.J.; Goun E.A. Noninvasive imaging and quantification of bile salt hydrolase activity: from bacteria to humans. Sci. Adv. 2021, 7(6), eaaz9857, DOI: 10.1126/sciadv.aaz9857 (link).
Cui, L.; Gouw, A.M.; LaGory, E.L.; Guo, S.; Attarwala, N.; Tang, Y.; Qi, J.; Chen, Y.S.; Gao, Z.; Casey, K.M.; Bazhin, A.A.; Chen, M.; Hu, L.; Xie, J.; Fang, M.; Zhang, C.; Zhu.; Wang, Z.; Giaccia, A.J.; Gambhir, S.S.; Zhu, W.; Felsher, D.W.; Pegram, M.D.; Goun, E.A.; Le, A.; Rao, J., Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat. Biotechnol. 2021, 39(3), 357-367. doi: 10.1038/s41587-020-0707-9 (link).
Bazhin AA, Sinisi R, De Marchi U, Hermant A, Sambiagio N, Maric T, Budin G, Goun EA. A bioluminescent probe for longitudinal monitoring of mitochondrial membrane potential. Nat. Chem. Biol. 2020, 16(12):1385-1393. doi: 10.1038/s41589-020-0602-1. Epub 2020 Aug 10. PubMed PMID: 32778841 (link).
Karatas H, Maric T, D'Alessandro PL, Yevtodiyenko A, Vorherr T, Hollingworth GJ, Goun EA. Real-Time Imaging and Quantification of Peptide Uptake in Vitro and in Vivo. ACS Chem. Biol. 2019,18;14(10):2197-2205. doi: 10.1021/acschembio.9b00439. Epub 2019 Sep 24. PubMed PMID: 31498986 (link).
Miyazaki T, Gharib SA, Hsu YA, Xu K, Khodakivskyi P, Kobayashi A, Paragas J, Klose AD, Francis KP, Dubikovskaya E, Page-McCaw PS, Barasch J, Paragas N. Cell-specific image-guided transcriptomics identifies complex injuries caused by ischemic acute kidney injury in mice. Commun. Biol. 2019, 2:326. doi: 10.1038/s42003-019-0571-7. eCollection 2019. PubMed PMID: 31508501; PubMed Central PMCID: PMC6718519 (link).
Maric T, Mikhaylov G, Khodakivskyi P, Bazhin A, Sinisi R, Bonhoure N, Yevtodiyenko A, Jones A, Muhunthan V, Abdelhady G, Shackelford D, Goun E. Bioluminescent-based imaging and quantification of glucose uptake in vivo. Nat. Methods 2019, 16(6):526-532. doi: 10.1038/s41592-019-0421-z. Epub 2019 May 13. PubMed PMID: 31086341; PubMed Central PMCID: PMC6546603 (link).
Marx V. Elena Goun. Nat. Methods 2019 Jun;16(6):449. doi: 10.1038/s41592-019-0429-4. PubMed PMID: 31086340.
Mermod M, Bongiovanni M, Petrova T, Goun E, Simon C, Tolstonog G, Monnier Y. Prediction of Occult Lymph Node Metastasis in Head and Neck Cancer with CD31 Vessel Quantification. Otolaryngol Head Neck Surg. 2019,160(2):277-283. doi: 10.1177/0194599818791779. Epub 2018 Aug 7. PubMed PMID: 30084319 (link).
Bazhin AA, Chambon M, Vesin J, Bortoli J, Collins JW, Turcatti G, Chou CJ, Goun EA. A Universal Assay for Aminopeptidase Activity and Its Application for Dipeptidyl Peptidase-4 Drug Discovery. Anal. Chem. 2019, 91(1):1098-1104. doi: 10.1021/acs.analchem.8b04672. Epub 2018 Dec 17. PubMed PMID: 30511572 (link).
Godinat A, Bazhin AA, Goun EA. Bioorthogonal chemistry in bioluminescence imaging. Drug Discov Today. 2018, 23(9):1584-1590. doi: 10.1016/j.drudis.2018.05.022. Epub 2018 May 18. Review. PubMed PMID: 29778694.
Reber J, Willershäuser M, Karlas A, Paul-Yuan K, Diot G, Franz D, Fromme T, Ovsepian SV, Bézière N, Dubikovskaya E, Karampinos DC, Holzapfel C, Hauner H, Klingenspor M, Ntziachristos V. Non-invasive Measurement of Brown Fat Metabolism Based on Optoacoustic Imaging of Hemoglobin Gradients. Cell Metab. 2018, 27(3):689-701.e4. doi: 10.1016/j.cmet.2018.02.002. PubMed PMID: 29514074.
Mezzanotte L, van 't Root M, Karatas H, Goun EA, Löwik CWGM. In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol. 2017, 35(7):640-652. doi: 10.1016/j.tibtech.2017.03.012. Epub 2017 May 10. Review. PubMed PMID: 28501458.
Wang Y, Thompson JM, Ashbaugh AG, Khodakivskyi P, Budin G, Sinisi R, Heinmiller A, van Oosten M, van Dijl JM, van Dam GM, Francis KP, Bernthal NM, Dubikovskaya EA, Miller LS. Preclinical Evaluation of Photoacoustic Imaging as a Novel Noninvasive Approach to Detect an Orthopaedic Implant Infection. J. Am. Acad. Orthop. Surg. 2017, 25 Suppl 1:S7-S12. doi: 10.5435/JAAOS-D-16-00630. PubMed PMID: 27941556; PubMed Central PMCID: PMC6056014.
Park HM, Russo KA, Karateev G, Park M, Dubikovskaya E, Kriegsfeld LJ, Stahl A. A System for In Vivo Imaging of Hepatic Free Fatty Acid Uptake. Gastroenterology. 2017, 152(1):78-81.e2. doi: 10.1053/j.gastro.2016.10.002. Epub 2016 Oct 11. PubMed PMID: 27742378; PubMed Central PMCID: PMC5164972.
Mermod M, Bongiovanni M, Petrova TV, Dubikovskaya EA, Simon C, Tolstonog G, Monnier Y. Correlation between podoplanin expression and extracapsular spread in squamous cell carcinoma of the oral cavity using subjective immunoreactivity scores and semiquantitative image analysis. Head Neck. 2017, 39(1):98-108. doi: 10.1002/hed.24537. Epub 2016 Jul 20. PubMed PMID: 27437903.
Stammes MA, Knol-Blankevoort VT, Cruz LJ, Feitsma HR, Mezzanotte L, Cordfunke RA, Sinisi R, Dubikovskaya EA, Maeda A, DaCosta RS, Bierau K, Chan A, Kaijzel EL, Snoeks TJ, van Beek ER, Löwik CW. Pre-clinical Evaluation of a Cyanine-Based SPECT Probe for Multimodal Tumor Necrosis Imaging. Mol Imaging Biol. 2016, 18(6):905-915. doi: 10.1007/s11307-016-0972-7. PubMed PMID: 27277828; PubMed Central PMCID: PMC5093207.
Stammes MA, Maeda A, Bu J, Scollard DA, Kulbatski I, Medeiros PJ, Sinisi R, Dubikovskaya EA, Snoeks TJ, van Beek ER, Chan AB, Löwik CW, DaCosta RS. The Necrosis-Avid Small Molecule HQ4-DTPA as a Multimodal Imaging Agent for Monitoring Radiation Therapy-Induced Tumor Cell Death. Front. Oncol. 2016, 6:221. doi: 10.3389/fonc.2016.00221. eCollection 2016. PubMed PMID: 27818949; PubMed Central PMCID: PMC5073092.
Mermod M, Bongiovanni M, Petrova TV, Dubikovskaya EA, Simon C, Tolstonog G, Monnier Y. Prediction of occult lymph node metastasis in squamous cell carcinoma of the oral cavity and the oropharynx using peritumoral Prospero homeobox protein 1 lymphatic nuclear quantification. Head Neck. 2016, 38(9):1407-15. doi: 10.1002/hed.24452. Epub 2016 Apr 4. PubMed PMID: 27043718.
Homulle HA, Powolny F, Stegehuis PL, Dijkstra J, Li DU, Homicsko K, Rimoldi D, Muehlethaler K, Prior JO, Sinisi R, Dubikovskaya E, Charbon E, Bruschini C. Compact solid-state CMOS single-photon detector array for in vivo NIR fluorescence lifetime oncology measurements. Biomed Opt. Express. 2016, 7(5):1797-814. doi: 10.1364/BOE.7.001797. eCollection 2016 May 1. PubMed PMID: 27231622; PubMed Central PMCID: PMC4871082.
Tharp KM, Jha AK, Kraiczy J, Yesian A, Karateev G, Sinisi R, Dubikovskaya EA, Healy KE, Stahl A. Matrix-Assisted Transplantation of Functional Beige Adipose Tissue. Diabetes. 2015, 64(11):3713-24. doi: 10.2337/db15-0728. Epub 2015 Aug 20. PubMed PMID: 26293504; PubMed Central PMCID: PMC4613967.
Vorobyeva AG, Stanton M, Godinat A, Lund KB, Karateev GG, Francis KP, Allen E, Gelovani JG, McCormack E, Tangney M, Dubikovskaya EA. Development of a Bioluminescent Nitroreductase Probe for Preclinical Imaging. PLoS One. 2015, 10(6):e0131037. doi: 10.1371/journal.pone.0131037. eCollection 2015. PubMed PMID: 26110789; PubMed Central PMCID: PMC4482324.
Geissbuehler S, Sharipov A, Godinat A, Bocchio NL, Sandoz PA, Huss A, Jensen NA, Jakobs S, Enderlein J, Gisou van der Goot F, Dubikovskaya EA, Lasser T, Leutenegger M. Live-cell multiplane three-dimensional super-resolution optical fluctuation imaging. Nat. Commun. 2014, 5:5830. doi: 10.1038/ncomms6830. PubMed PMID: 25518894; PubMed Central PMCID: PMC4284648.
Godinat A, Budin G, Morales AR, Park HM, Sanman LE, Bogyo M, Yu A, Stahl A, Dubikovskaya EA. A biocompatible "split luciferin" reaction and its application for non-invasive bioluminescent imaging of protease activity in living animals. Curr. Protoc. Chem. Biol. 2014, 6(3):169-189. doi: 10.1002/9780470559277.ch140047. PubMed PMID: 25205565; PubMed Central PMCID: PMC4219325.
Dubikovskaya E, Chudnovskiy R, Karateev G, Park HM, Stahl A. Measurement of long-chain fatty acid uptake into adipocytes. Methods Enzymol. 2014, 538:107-34. doi: 10.1016/B978-0-12-800280-3.00007-4. PubMed PMID: 24529436; PubMed Central PMCID: PMC4269161.
Godinat A, Park HM, Miller SC, Cheng K, Hanahan D, Sanman LE, Bogyo M, Yu A, Nikitin GF, Stahl A, Dubikovskaya EA. A biocompatible in vivo ligation reaction and its application for noninvasive bioluminescent imaging of protease activity in living mice. ACS Chem. Biol. 2013, 8(5):987-99. doi: 10.1021/cb3007314. Epub 2013 Mar 29. PubMed PMID: 23463944; PubMed Central PMCID: PMC3836283.
Henkin AH, Cohen AS, Dubikovskaya EA, Park HM, Nikitin GF, Auzias MG, Kazantzis M, Bertozzi CR, Stahl A. Real-time noninvasive imaging of fatty acid uptake in vivo. ACS Chem. Biol. 2012, 7(11):1884-91. doi: 10.1021/cb300194b. Epub 2012 Sep 6. PubMed PMID: 22928772; PubMed Central PMCID: PMC3500440.
Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang CJ. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc. Natl. Acad. Sci. USA 2010, 107(50):21316-21. doi: 10.1073/pnas.1012864107. Epub 2010 Nov 29. PubMed PMID: 21115844; PubMed Central PMCID: PMC3003011.
Cohen AS, Dubikovskaya EA, Rush JS, Bertozzi CR. Real-time bioluminescence imaging of glycans on live cells. J. Am. Chem. Soc. 2010, 132(25):8563-5. doi: 10.1021/ja101766r. PubMed PMID: 20527879; PubMed Central PMCID: PMC2890245.
Dubikovskaya EA, Thorne SH, Pillow TH, Contag CH, Wender PA. Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proc. Natl. Acad. Sci. USA 2008, 105(34):12128-33. doi: 10.1073/pnas.0805374105. Epub 2008 Aug 19. PubMed PMID: 18713866; PubMed Central PMCID: PMC2527877.
Lee HL, Dubikovskaya EA, Hwang H, Semyonov AN, Wang H, Jones LR, Twieg RJ, Moerner WE, Wender PA. Single-molecule motions of oligoarginine transporter conjugates on the plasma membrane of Chinese hamster ovary cells. J. Am. Chem. Soc. 2008, 130(29):9364-70. doi: 10.1021/ja710798b. Epub 2008 Jun 26. PubMed PMID: 18578528; PubMed Central PMCID: PMC2725020.
Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. The design of guanidinium-rich transporters and their internalization mechanisms. Adv. Drug. Deliv. Rev. 2008, 60(4-5):452-72. doi: 10.1016/j.addr.2007.10.016. Epub 2007 Nov 9. Review. PubMed PMID: 18164781; PubMed Central PMCID: PMC2533582.
Wender PA, Goun EA, Jones LR, Pillow TH, Rothbard JB, Shinde R, Contag CH. Real-time analysis of uptake and bioactivatable cleavage of luciferin-transporter conjugates in transgenic reporter mice. Proc. Natl. Acad. Sci. USA 2007, 104(25):10340-5. doi: 10.1073/pnas.0703919104. Epub 2007 Jun 11. PubMed PMID: 17563383; PubMed Central PMCID: PMC1965515.
Goun EA, Pillow TH, Jones LR, Rothbard JB, Wender PA. Molecular transporters: synthesis of oligoguanidinium transporters and their application to drug delivery and real-time imaging. Chembiochem. 2006, 7(10):1497-515. doi: 10.1002/cbic.200600171. Review. PubMed PMID: 16972294.
Jones LR, Goun EA, Shinde R, Rothbard JB, Contag CH, Wender PA. Releasable luciferin-transporter conjugates: tools for the real-time analysis of cellular uptake and release. J. Am. Chem. Soc. 2006, 128(20):6526-7. doi: 10.1021/ja0586283. PubMed PMID: 16704230.
Goun EA, Shinde R, Dehnert KW, Adams-Bond A, Wender PA, Contag CH, Franc BL. Intracellular cargo delivery by an octaarginine transporter adapted to target prostate cancer cells through cell surface protease activation. Bioconjug Chem. 2006, 17(3):787-96. doi: 10.1021/bc0503216. PubMed PMID: 16704219.
Goun E, Cunningham G, Chu D, Nguyen C, Miles D. Antibacterial and antifungal activity of Indonesian ethnomedical plants. Fitoterapia 2003, 74(6):592-6. doi: 10.1016/s0367-326x(03)00117-5. PubMed PMID: 12946723.
Goun E, Cunningham G, Solodnikov S, Krasnykch O, Miles H. Antithrombin activity of some constituents from Origanum vulgare. Fitoterapia 2002, 73(7-8):692-4. doi: 10.1016/s0367-326x(02)00245-9. PubMed PMID: 12490231.
Goun EA, Petrichenko VM, Solodnikov SU, Suhinina TV, Kline MA, Cunningham G, Nguyen C, Miles H. Anticancer and antithrombin activity of Russian plants. J. Ethnopharmacol. 2002, 81(3):337-42. doi: 10.1016/s0378-8741(02)00116-2. PubMed PMID: 12127234.